IPO Intellectual Property Observation | How to Discuss Continuous R&D Capability under the Authorization Introduction Model
Release Date:2024-02-06 Number of views:151
introduction
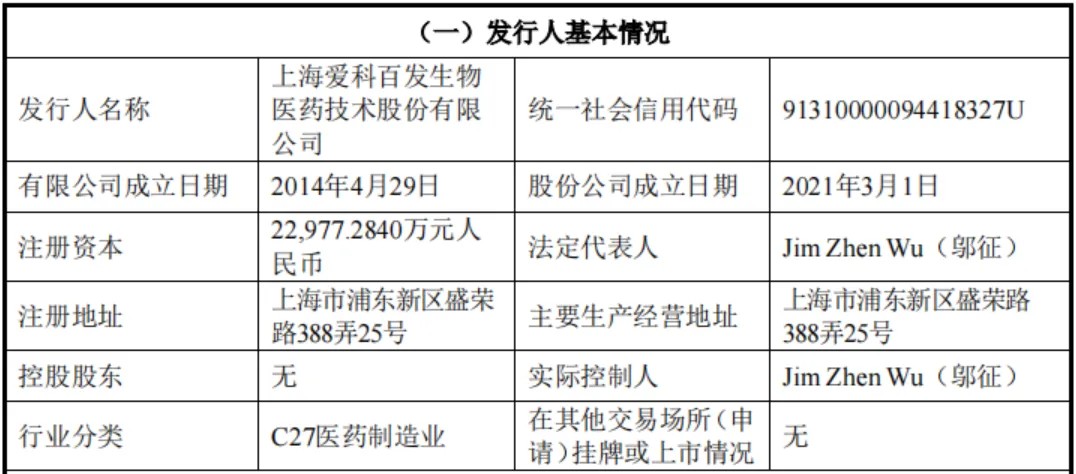
On January 8, 2024, Shanghai Aike Baifa Biomedical Technology Co., Ltd. (referred to as "Aike Baifa") terminated its listing on the Science and Technology Innovation Board. According to the prospectus, the company is a biopharmaceutical enterprise that is about to enter the commercialization stage and focuses on global innovative drug research and development in the fields of pediatric diseases, respiratory system and lung diseases. Since the company launched its IPO on April 20, 2023, it has undergone one round of inquiries, during which it has been repeatedly questioned about its ongoing research and development capabilities.

1、 Under the authorized introduction mode, research and development capabilities are questioned
According to the application materials, there are currently five drugs under Phase I clinical research and later stages of Aike Baifa. Among them, three products, including the core product AK0529, are authorized imports, and one independently developed product has the same active ingredient as the authorized imported product. In addition, as disclosed in the prospectus, the actual controller of the issuer, JIMCHENWU (Wu Zheng), has previously served as the head of Roche (China)'s biological and viral departments, leading the development of the new anti RSV drug AK0529 during his tenure at Roche. After acquiring Genentech Inc. in 2013, Roche made strategic adjustments and changed its positioning for the development of RSV pipelines. Therefore, in 2013, JIMZHENWU (Wu Zheng) resigned and established Aike Baifa. In 2014, Roche obtained the global equity of AK0529 to continue research and development.

As is well known, new drug research and development is a systematic project with large investment, long cycle, and high risk. Many pharmaceutical companies (especially innovative drug companies) choose to introduce technology through licensing and carry out digestion, absorption, clinical trials, etc. on this basis in order to expand their product pipeline and promote a highly attractive new drug research and development project at relatively low cost, thereby increasing the probability of successful drug development; However, this also means that the original technology research and development is not within the company, which may question the company's sustained research and development capabilities. Due to the fact that the Science and Technology Innovation Board, as a testing ground for "hard technology," places greater emphasis on the issuer's key core technologies and research and development innovation capabilities, the review committee attaches greater importance to matters related to the introduction of technology through authorization and licensing.
In response to this, the audit committee requested that Aike Baifa explain in what aspects the company's core competitiveness is mainly reflected in the authorized introduction model, whether it is mainly reflected in promoting clinical research of related products, whether the company's new drug research and development, production, and sales rely on third parties, the specific role of the issuer's team in the research and development of authorized introduced compounds and preclinical research, and whether there are substantial improvements and innovations in the existing achievements of the original research party.

The audit committee is more concerned about the role and importance of promoting clinical research on related products, clinical research and development strategies, and program design in the process of authorizing the introduction of products into the company's clinical research. However, the response from Aike Baifa only discusses the promotion of clinical research strategies, program design, and other aspects of clinical research, without considering the actual situation of promoting related products and the importance of clinical research, clinical research and development strategies, and program design for authorizing the introduction of products, which cannot reflect the company's core competitiveness in clinical research.

Although Aike Baifa also stated in the final summary that it has demonstrated core competitiveness in source innovation and drug discovery, efficacy and toxicology research, pharmaceutical research, etc., it was not elaborated in detail in the main text.
Before discussing the core competitiveness of a company, the issuer first needs to identify the company's own core competencies. The characteristics of core competitiveness are valuable, scarce, irreplaceable, difficult to imitate, and constantly changing in a dynamic upward trend. For science and technology innovation enterprises, the demonstration of their core competitiveness should be considered from the following perspectives: ① What resources and capabilities related to technological innovation do enterprises possess, such as intellectual property intangible assets, research and development innovation capabilities, achievement transformation capabilities, core technologies, research and development systems, etc. ② What are the competitive advantages compared with comparable companies, such as intellectual property reserves, progressiveness of core technologies, and sustainability of R&D and innovation capabilities. ③ Whether the development direction of core technology conforms to the national policy trend and industry conventions.
Secondly, after clarifying the direction of demonstration, we should also further consider how to select demonstration indicators, because core competitiveness such as R&D innovation ability, achievement transformation ability and technological progressiveness are usually not quantifiable. If just like the above description of AGCO Baifa, "the core competitiveness of the company is not only reflected in the company's solid and professional clinical development ability, professional R&D team and strong competitiveness in..., but also reflected in...", then the core competitiveness of the enterprise cannot be demonstrated, and it is difficult to convince the audit committee and investors. Therefore, enterprises should use quantifiable and concrete science and technology innovation indicators as much as possible for demonstration.
Finally, the demonstration of a company's core competitiveness is a systematic project. When discussing the core competitiveness of R&D innovation capability, it is not only necessary to combine multiple aspects such as R&D personnel, R&D salaries, R&D projects, R&D achievements, and intellectual property reserves; It is even more important to mutually verify multiple quantifiable indicators, such as matching indicators such as the number of patents, patent layout, and inventor situation of the main products with indicators such as research and development achievements, projects, and personnel, to demonstrate the R&D innovation capability of the enterprise from multiple perspectives.
2、 Does the R&D result infringe upon rights under the authorized introduction mode
If science and technology innovation enterprises do not conduct sufficient due diligence during the research and development process, it is inevitable that they will infringe on the patent rights of others. Especially for the authorized introduction model, when the technology source is external introduction combined with independent research and development, how to properly divide the technology and avoid infringement risks is a key issue that science and technology innovation enterprises should consider.


One of the core products of Aike Baifa, AK3280, was globally licensed by Genentech in 2018 Inc., Intermune. Inc., and F. Hoffmann La Roche Ltd. grant the issuer authorization, at the time when the original research party had completed a Phase I clinical trial in the UK. Due to the external introduction and independent research and development of this technology, the audit committee questions whether its independently developed products AK0707 and AK3280 have the same or similar compound structure, crystal form, target of action, mechanism, etc., as well as whether there is a risk of infringement in AK0707's patents and other research and development achievements.

According to the inquiry reply letter, the issuer's response to the risk of AK0707 patent infringement was only based on the "Memorandum on AK0707 Patent Infringement Risk Issues" issued by the law firm to demonstrate whether it was infringing, and only discussed that the chemical structure, crystal form, and indications of the target compound, including AK0707, did not fall within the scope of protection of AK3280 related patents. There are several shortcomings in the response here:
Firstly, when arguing whether there is infringement, only the "Memorandum on the Risk of AK0707 Patent Infringement Issues" was provided, without presenting the infringement analysis of the target compound and related patents in the form of technical feature comparison, nor providing detailed reasons for the non infringement of the chemical structure, crystal form, and indications of the target compound;
Secondly, the implementation of AK0707 may involve multiple patented technologies. Therefore, when issuing a patent infringement comparison opinion, a comprehensive freedom to operate (FTO) investigation should be conducted based on AK0707's specific target market (such as Japan, the United States, Europe, and other countries or regions), in order to draw a comprehensive and prudent patent infringement analysis conclusion.
If Aike Baifa has conducted comprehensive patent searches and free implementation due diligence before the introduction of technology authorization, fully analyzing the patent infringement risks and competitive status of the company's related projects, the response during the IPO process will be more comprehensive and complete.
conclusion
Qiu Yong, Chairman of the Shanghai Stock Exchange, stated at the 14th Lujiazui Forum that the capital market plays a unique and important role in serving technological innovation. Since the opening of the Science and Technology Innovation Board, it has also become a gathering place for "hard technology" enterprises, playing an important role in promoting the effective connection between technology innovation oriented enterprises and capital. R&D innovation capability is the core competitiveness of scientific and technological innovation oriented enterprises for sustainable and stable development. As an important index element for the listing of scientific and technological content of enterprises and the Science and Technology Innovation Board, especially for innovative pharmaceutical enterprises, it is necessary to ensure the progressiveness and independence of enterprise core technologies.
Enterprises should strive to form their core technologies through independent research and development, while ensuring the correspondence between core technologies and their main drugs; Patents that have a significant impact on core technology or operating revenue should be avoided as much as possible through cooperative development, commissioned development, or the formation of shared patents, in order to maintain the independence of core technology; If there is an authorization introduction model similar to that of Aike Baifa, their respective rights and obligations should also be clarified through agreements in advance to avoid potential ownership disputes becoming obstacles in the listing or financing process, or affecting the stability of the company's core technology, which may have an impact on the sustainability of the company's future production and operation.
- Prev:Judgment of "Modification Exceeding Scope" of Design Patent in Patent Invalidation Procedure - A Brief Analysis of the Request for Invalidation of "Lamp" Design Patent
- Next:'dissimilarity 'can also constitute' similarity '-' using the spear of the child to attack the shield of the child 'is repeated











 Nanjing
Nanjing Suzhou
Suzhou Changzhou
Changzhou Hefei
Hefei Hangzhou
Hangzhou